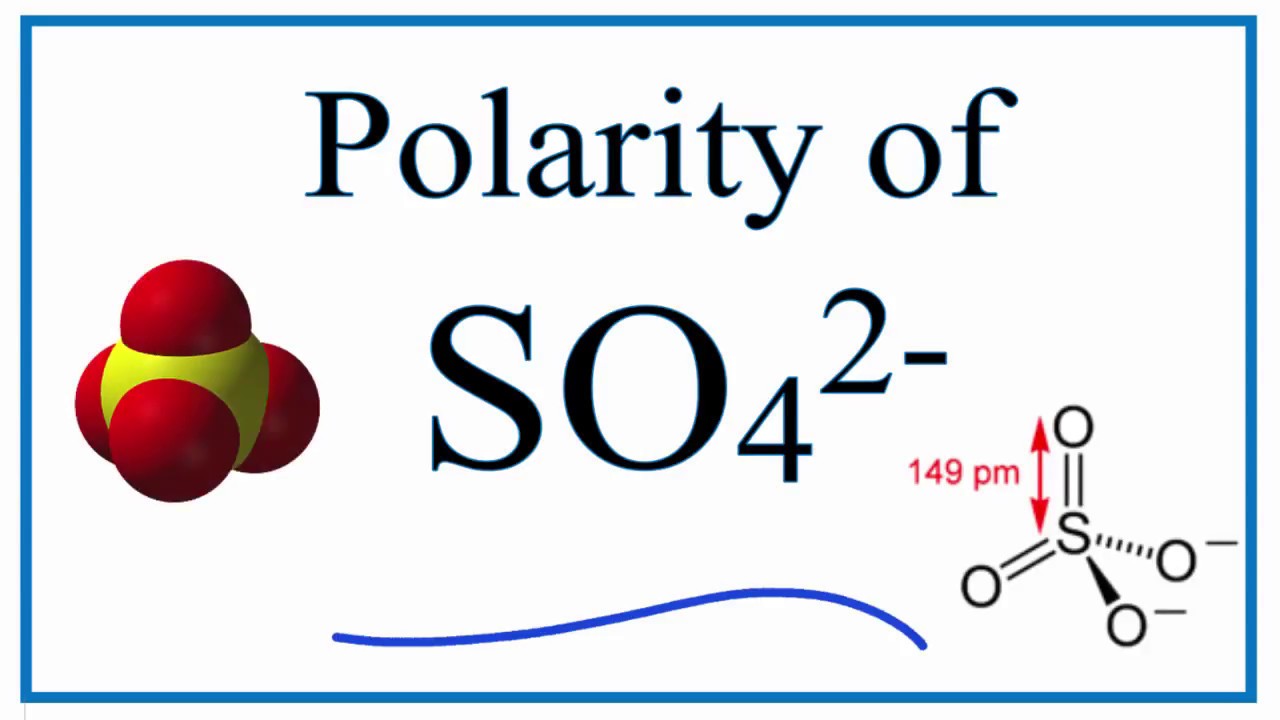
Is SO42 Polar or Nonpolar? (sulfate ion) YouTube
Before we dive into the polar or nonpolar debate, let's first define the molecular makeup of SO4 2-. SO4 2- is a polyatomic ion consisting of one sulfur atom and four oxygen atoms. The overall charge of the ion is negative two since oxygen has a higher electronegativity than sulfur. So42- Polar or Nonpolar: The Answer. The answer to whether.

So42 Lewis Structure Molecular Geometry slidesharetrick
Sulfate ion (SO42-) is one of the most common ions that people in chemistry need to deal with. This is a polyatomic anion having a negative charge of -2. We find sulfates in a wide range of compounds, some of the well-known being MgSO4, CaSO4, Na2SO4, and PbSO4. We can easily prepare sulfates via oxidizing metal sulfites and sulfides.

Hướng dẫn vẽ cấu trúc Lewis của so4 2 lewis structure chi tiết và dễ hiểu
The SOF4 lewis structure consists of sulphur, oxygen and fluorine atoms having 6, 6 and 7 electrons respectively. Therefore, total valence electrons in SOF4 molecule is 6 (S) + 6 (O) + 7 x 4 (F) = 40. Hence SOF4 molecule has total forty valence electrons present on it. Also if we calculate total electron pairs of SOF4 then 40 / 2 = 20, we have.

Is SF4 Polar or Nonpolar? (Sulfur Tetrafluoride) YouTube
Answer = SO4 2- ( sulfate ) is Nonpolar What is polar and non-polar? Polar "In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms.

Best Overview Is SO2 Polar or Nonpolar Science Education and Tutorials
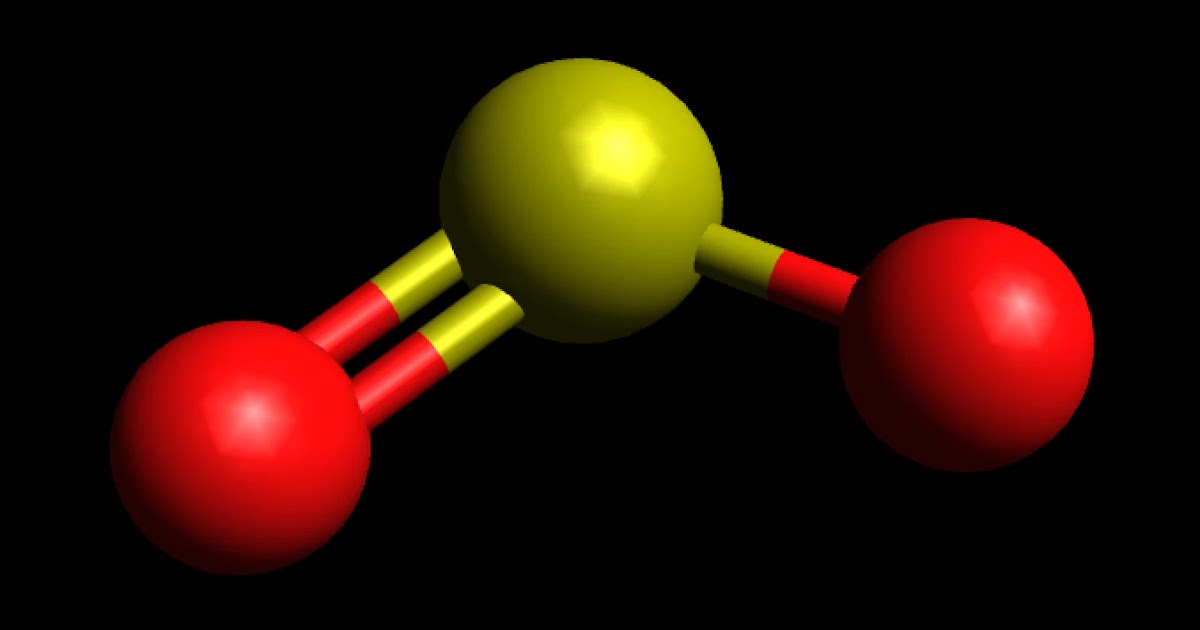
To determine if SO 2 (sulfur dioxide) is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here. Sulfur is the central atom, so we can draw the skeletal structure:
Chf3 Polar Or Non Polar
Figure \(\PageIndex{6}\): The molecular geometry of a molecule affects its polarity. CO 2 is a nonpolar molecule, while H 2 O is a polar molecule. It should be noted that if the all of the bonds in a molecule are nonpolar, then the molecule will be nonpolar. Consider a molecule of O 2, . The double bond between the two oxygen atoms is a.
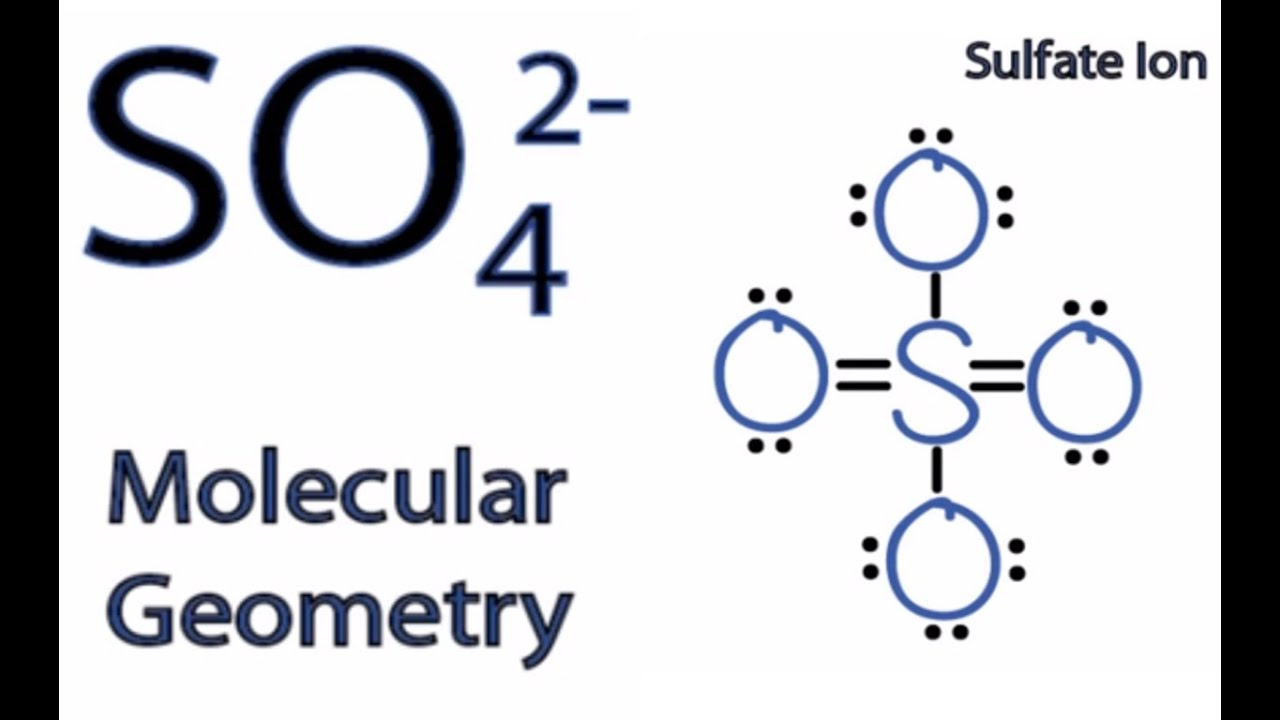
SO4 2 Molecular Geometry / Shape and Bond Angles YouTube
SO42- is a chemical formula for Sulfate ion; it comprises one Sulfur Atom and four oxygen atoms. It also has a -2 charge because of the additional electrons it accepts to attain this structure. This blog post will help you understand if this ion is polar or nonpolar, although a -2 charge might confuse you.

Is SO2 Polar or Nonpolar? Techiescientist
Learn to determine if SO42- is polar or nonpolar based on the polarity between bonds and the molecular geometry (shape).Ions, like SO42- (sulfate) are someti.

Is SO2 Polar or Nonpolar? Techiescientist
Learn to determine if SO2 (Sulfur dioxide) is polar or non-polar based on the Lewis Structure and the molecular geometry (shape).We start with the Lewis Stru.

SO42 Lewis Structure, Molecular Geometry, Hybridization, and Polarity
Draw the most important Lewis structure for [ SO4 ]2− and then answer the following questions. The underlined atom is the central atom. All other atoms are bonded directly to the central atom. (a) What is the electron-group geometry, according to VSEPR theory? (b) What is the molecular geometry? (c) Is this species polar or nonpolar?

H2so4 Estrutura De Lewis ENSINO
SO4^2-: - The 3D sketch for SO4^2- is a tetrahedral shape, with bond angles of approximately 109.5 degrees. AsF5: - The 3D sketch for AsF5 is a trigonal bipyramidal shape, with bond angles of approximately 90 and 120 degrees. Answer Step 3: Finally, we need to determine if each molecule is polar or nonpolar.

Lewis Structure SO4 2 plus dipoles, shape, angles, resonance and
Is SO4 2- polar or nonpolar? Updated: 8/10/2023 Wiki User ∙ 16y ago Study now See answers (3) Best Answer Copy It is Nonpolar Wiki User ∙ 16y ago This answer is: More answers Wiki User ∙ 5y ago.
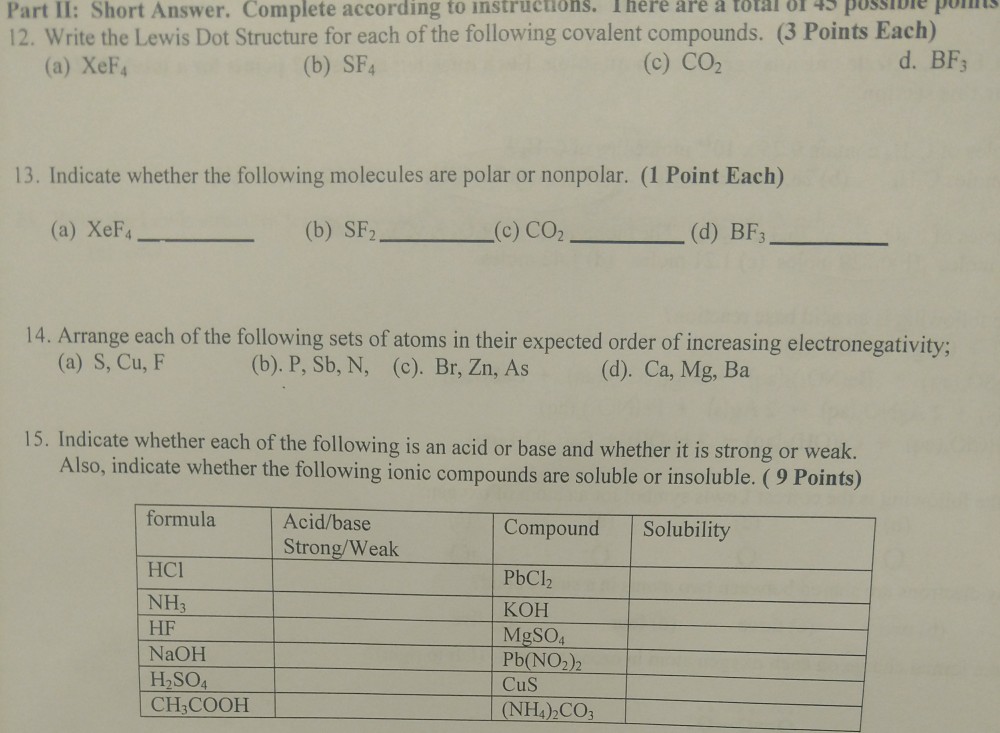
Solved Part II Short Answer. Complete according to
Sulfate is a very weak oxidizing agent. Since sulfur is in its maximum oxidation number in sulfate ion, this ion cannot act as a reducing agent. This page titled Sulfate Ion (SO₄²⁻) is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by James P. Birk. Sulfate ion is a very weak base.
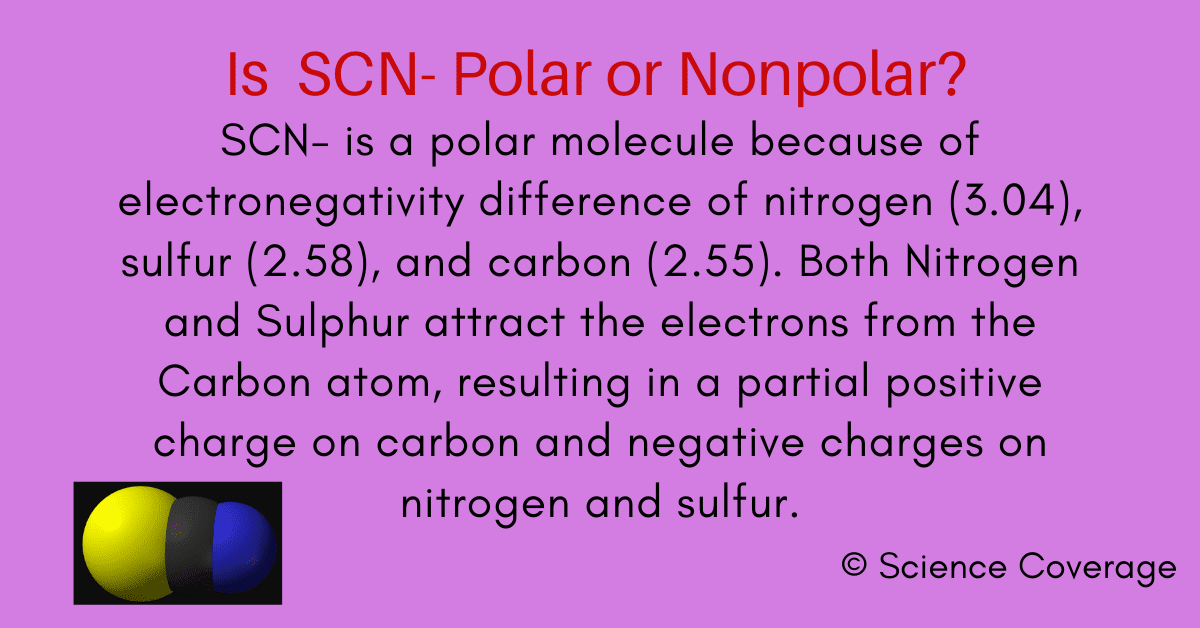
Is SCN Polar or Nonpolar?
SOX4X2− S O X 4 X 2 − is a polyatomic ion as well as a nonpolar covalent compound. How can it be ionic and covalent at the same time? Same applies to sulfite SOX3X2− S O X 3 X 2 −. It's a polyatomic ion as well as a polar covalent bond. How? bond ions covalent-compounds polarity Share Cite Improve this question Follow edited Apr 22, 2021 at 21:20
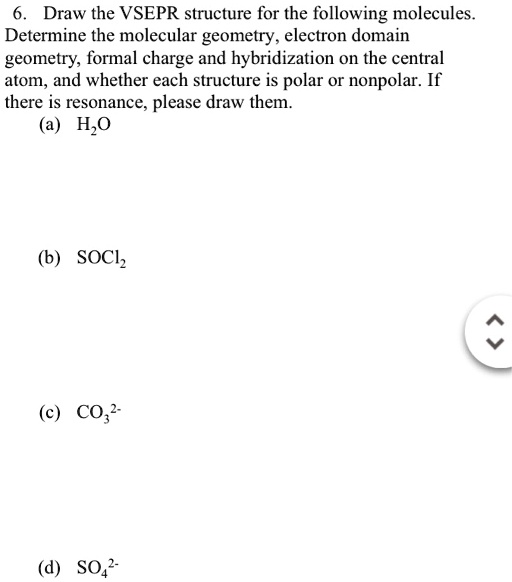
SOLVED Draw the VSEPR structure for the following molecules. Determine
SO42- is a chemical name for the sulfate ion. It comprises one Sulphur atom, four Oxygen atoms, and a charge of -2. It is a polyatomic anion and is used widely to synthesize other sulfates such as Zinc Sulfates, Magnesium sulfates, Iron sulfates, and much more. It is also a sulfate salt for sulphuric acids.
MakeTheBrainHappy Is SO2 Polar or Nonpolar?
Is SO42- Polar or Nonpolar? (Sulfate Ion) Geometry of Molecules 2.71K subscribers 5 679 views 1 year ago Polarity of Molecules Hello Guys! SO42- ion or Sulphate ion's polarity is quite.