
FileAcetone2Dskeletal.png Wikimedia Commons
Acetone Lewis structure is written with the atomic symbol of atoms in the molecule. The outer orbit electrons of a particular atom are shown by dot symbols around that particular atom. These electrons may forms bond with the outer shell electrons of the atom next to it.
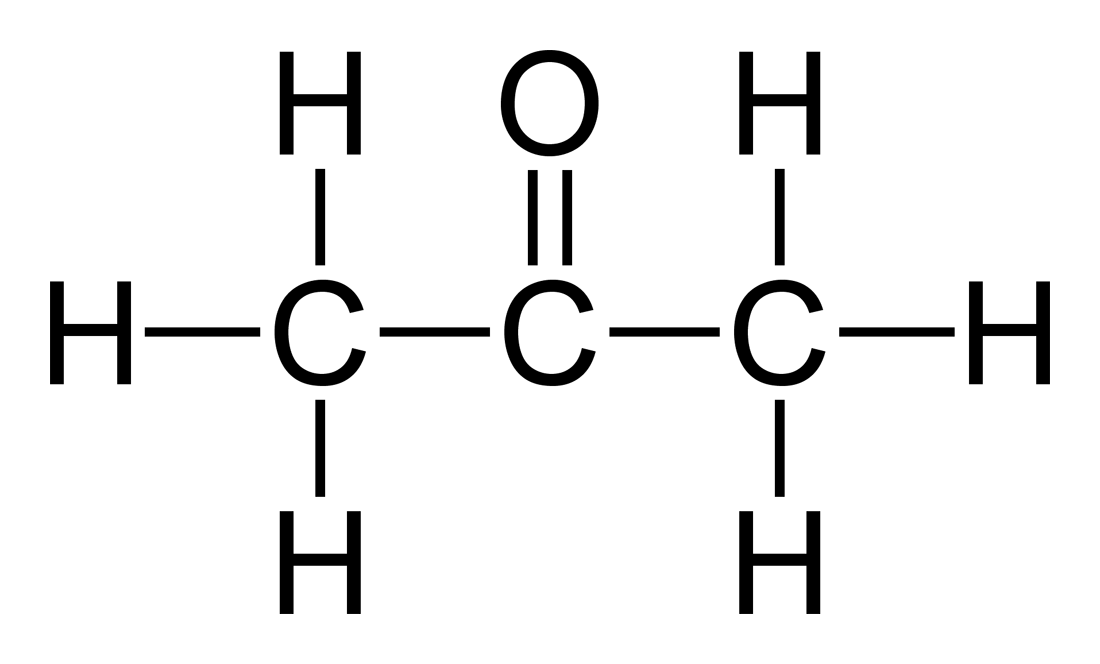
Acetone Formula (C3H6O) Structural and Organic Formula of Acetone (Propanone)
The Acetone Lewis structure is an example of an organic functional group called a Ketone. The Ketone functional group is made up on three Carbon atoms with an Oxygen atom double bonded to the center Carbon atom. The Lewis structure for Acetone is the simpliest Ketone possible.

Acetone Formula Properties, Preparation & More Embibe
The C3H6O Lewis structure is a molecular formula that represents a compound with three carbon atoms, six hydrogen atoms, and one oxygen atom. The structure of the molecule is such that the carbon atoms are arranged in a chain with the oxygen atom bonded to one of the end carbon atoms. Each carbon atom in the chain is also bonded to two hydrogen.

What are acetone? Definition, Types and Importance chemistry AESL
Draw a minor resonance structure for acetone (IUPAC name 2-propanone). Explain why it is a minor contributor. Are acetone and 2-propanol resonance contributors of each other? Explain. Exercise 2.18: Draw four additional resonance contributors for the molecule below. Label each one as major or minor (the structure below is of a major contributor).

a. draw the lewis structure of acetone, (CH3)2CO (C in the middle, with two C's and an O bonded
Lewis structure of Acetone (also known as Propanone or C3H6O) contains three Carbon atoms (C) in a row which have an Oxygen atom (O) attached to a central Carbon atom (C) forming a double bond. The outer Carbon atoms (C) are surrounded by the Hydrogen atoms (H). The Oxygen atom has 2 lone pairs.
Illustrated Glossary of Organic Chemistry Acetone
Geometry of Molecules 3.23K subscribers Subscribe Subscribed 4.3K views 1 year ago Lewis Structure CH3COCH3 is a chemical formula for acetone. And to help you understand the Lewis.

C3H6O Lewis Structure (Acetone) Functional group, Molecules, Lewis
The Lewis structure of acetone molecule is a way to present the outermost valence electrons that are involved in chemical bonding. C3H6O is a trigonal planer molecule. It consists of three carbon, six hydrogen, and one oxygen atoms.

Acetone Formula C3H6O Structure, Formula, Uses Embibe
Acetone | CH3COCH3 or CH3-CO-CH3 or C3H6O | CID 180 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.
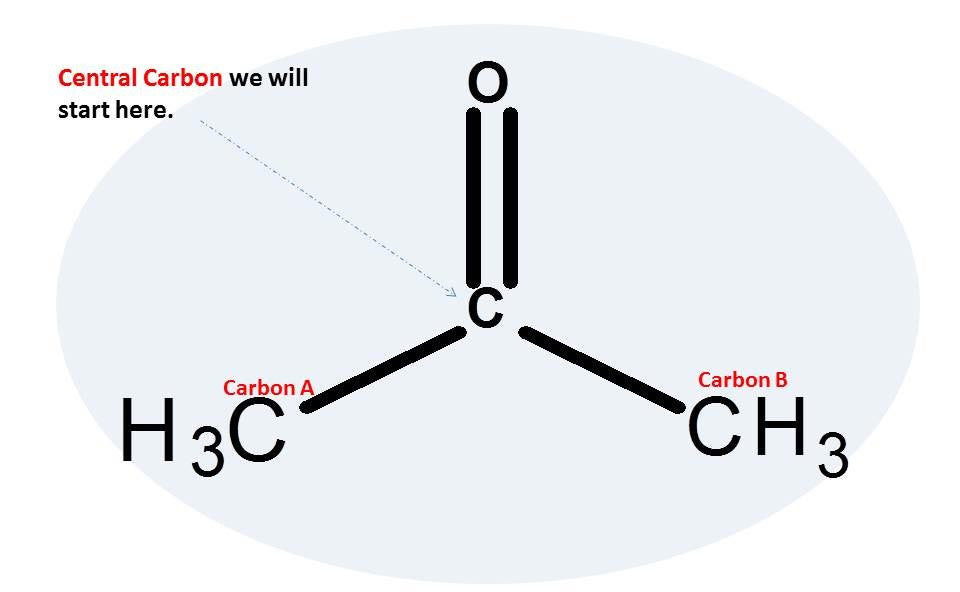
Acetone Duluth Labs
A step-by-step explanation of how to draw the Acetone Lewis Structure. Acetone is a member of the Ketone family of organic compounds. It is the simplest.

Acetone Formula Properties, Preparation & More Embibe
0:00 / 2:07 A step-by-step explanation of how to draw the (CH3)2CO Lewis Dot Structure (Acetone).For the (CH3)2CO structure use the periodic table to find the total numb.

Acetone Formula Properties, Preparation & More Embibe
Steps Here's how you can easily draw the acetone Lewis structure step by step: #1 Draw a rough skeleton structure #2 Mention lone pairs on the atoms #3 If needed, mention formal charges on the atoms #4 Minimize formal charges by converting lone pairs of the atoms, and try to get a stable Lewis structure

Acetone Formula C3H6O Structure, Formula, Uses Embibe
Steps of drawing Acetone (C3H6O) lewis structure Step 1: Find the total valence electrons in C3H6O molecule. In order to find the total valence electrons in a C3H6O molecule, first of all you should know the valence electrons present in carbon atom, hydrogen atom as well as oxygen atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.)
SIGMAALDRICH Acetone, 500mL, ACS Reagent, 99.5 Percent 45ZZ78179124500ML Grainger
For the Lewis structure for Acetone, calculate the total number of valence electrons for the Acetone molecule. After determining how many valence electrons there are in Acetone, place them around the central atom to complete the octets. There are a total of 24 valence electrons in the Lewis structure for Acetone. It is helpful if you:
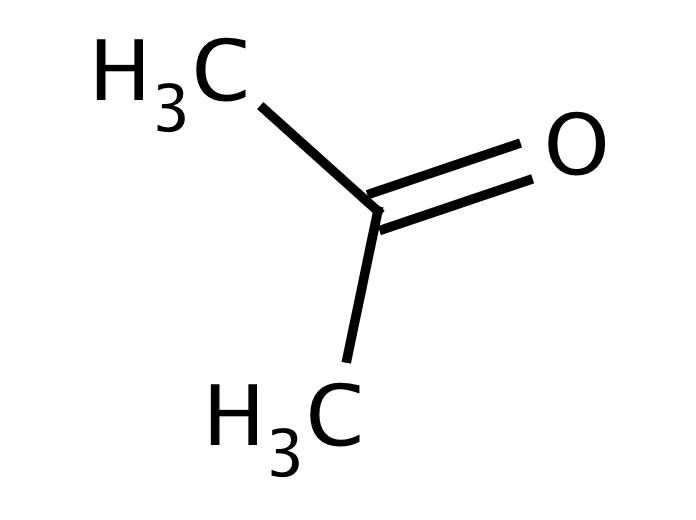
Purchase Acetone SG [67641] online • Catalog • Molekula Group
Geometry of Molecules. Molecular geometry, also known as the molecular structure, is the three-dimensional structure or arrangement of atoms in a molecule. Understanding the molecular structure of a compound can help determine the polarity, reactivity, phase of matter, color, magnetism, as well as the biological activity.

Chemistry Molecule Acetone
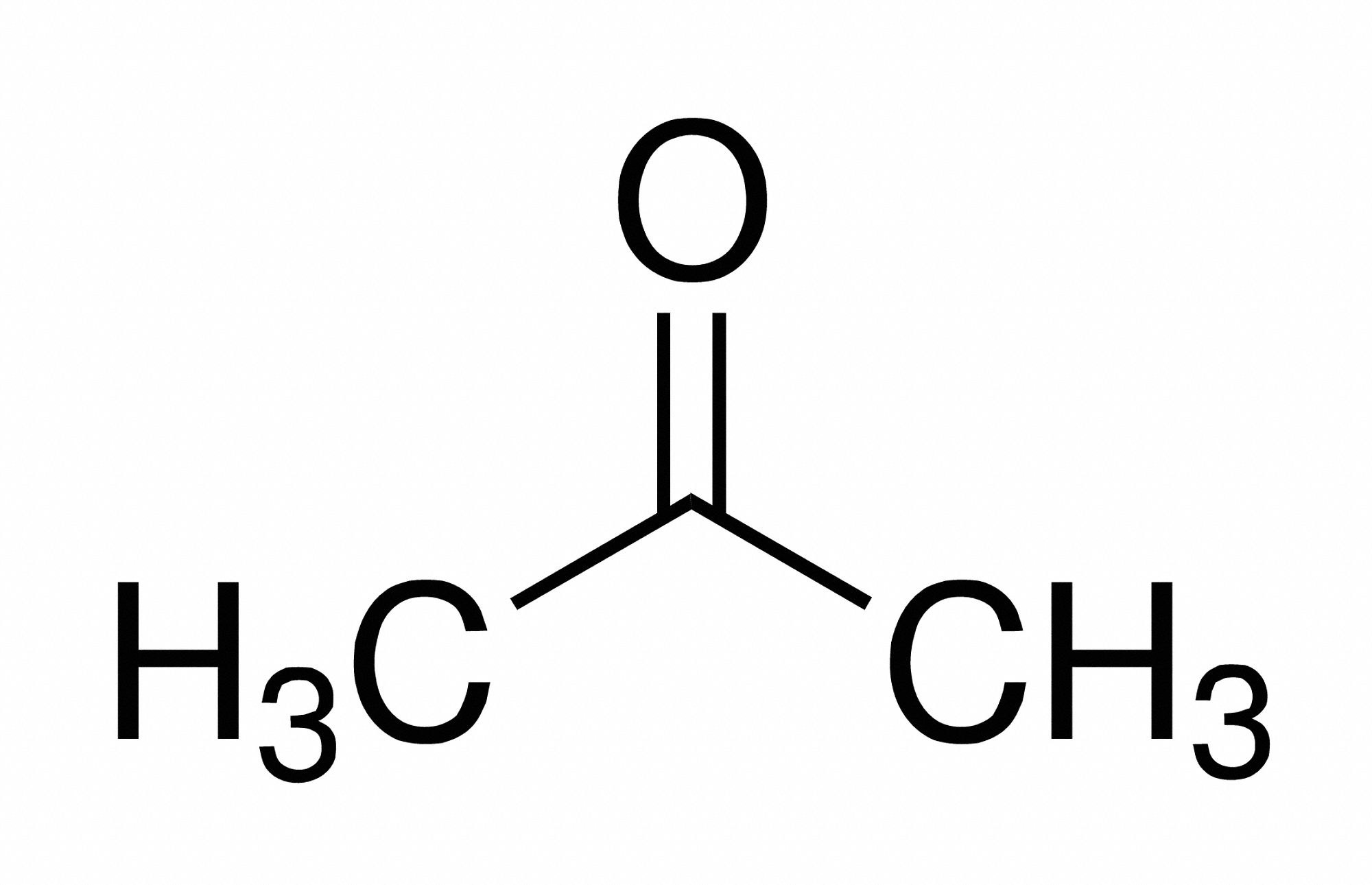
Acetone ( 2-propanone or dimethyl ketone) is an organic compound with the formula (CH3)2CO. [22] It is the simplest and smallest ketone ( >C=O ). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odor. Acetone is miscible with water and serves as an important organic solvent in industry, home, and laboratory.

Acetone Formula C3H6O Structure, Formula, Uses Embibe
Acetone: The simplest ketone . Also polar ( ε = 21) aprotic solvent of molecular formula C 3 H 6 O. Also called 2-propanone or di methyl ketone. Molecular Structure of Acetone